The EASE Study: Protecting Maternal Pelvic Health
What is the EASE Study?
The EASE study aims to evaluate whether an investigational device, called the “Materna Prep Device”, can make childbirth safer and less likely to cause injury to one of the pelvic floor muscles.
When a baby is born through the vagina, it can sometimes cause injuries to one of the muscles in the surrounding area. The muscle can get hurt when the baby’s head passes through the vagina, especially when it’s being pushed out. This injury can lead to problems after childbirth like pelvic organ prolapse which includes symptoms of vaginal pressure, or feeling a bulge or lump coming out of the vagina.
Right now, there is no proven way to prevent this injury. While there are some different techniques, such as massaging the area around the vagina or using hot packs, they vary a lot in how they’re done and when they’re used, and their effectiveness.
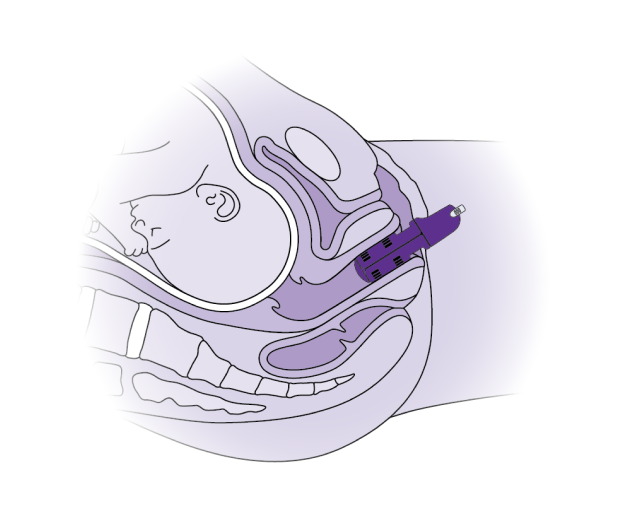
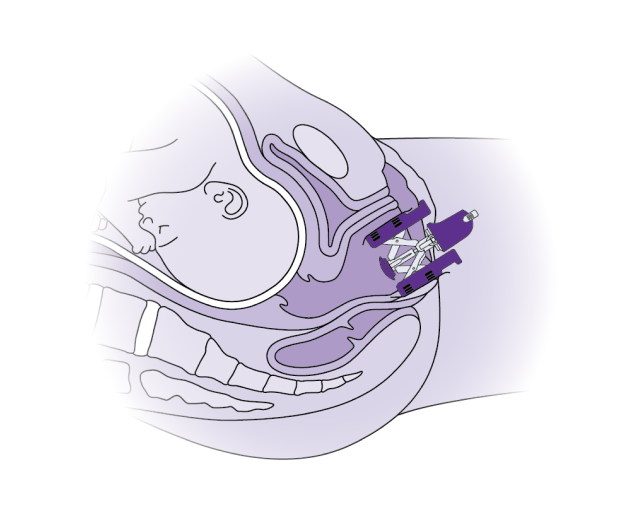
The Materna Prep Device is a tool used during the early stages of labor that gently stretches the vagina and surrounding muscles, which may reduce injury to one of the pelvic floor muscles surrounding the vagina. Essentially, it’s preparing your pelvic floor muscles to handle the strain of childbirth more smoothly, just as you would warm up your muscles before a workout.
Women pregnant with their first child planning for a vaginal birth at a participating hospital may be eligible with the following entry criteria:
- First-time mother, pregnant with one baby
- At least 18-years old
- Planning to get an epidural
Join the Research. Find a Site That’s Right for You!
What to Expect During the Study?
Day of Delivery
After you receive your epidural and before giving birth, you will be randomized to one of two groups:
- Participants who receive Materna Prep (device group), or
- Participants who deliver without use of Materna Prep (control group).
If you are assigned to the device group, Materna Prep will be inserted into your vagina and will gradually expand over about an hour. Before you start pushing, the device will be removed. If you are assigned to the control group, you will deliver without using Materna Prep.
After Delivery
Six weeks after delivery
Data will be collected for the study when you return for your routine 6-week postpartum visit.
Three months after delivery
You will return for a study visit with a unique ultrasound to check on your pelvic floor muscles
One year after delivery
You will return for a final study visit to have a unique ultrasound to check on your pelvic floor muscles.
Why participate in this study?
- Vaginal delivery is associated with pelvic floor trauma1
- Pelvic floor injury could lead to fecal incontinence and pelvic organ prolapse, two major forms of pelvic floor disorders2
- Women who vaginally deliver are 5x more likely to have prolapse3
Currently, there are no proven treatments to reduce or prevent pelvic floor damage during vaginal delivery.
1. Ashton-Miller, 2009 On the Biomechanics of Vaginal Birth and Common Sequelae. Annual Review of Biomedical Engineering, 11, 163-176. 2. S Mant, 1997, May. Epidemiology of genital prolapse: observations from the Oxford Family Panning Association Study. British Journal of Obstetrics and Gynaecology, 104(5), 579-585. 3.
2. S Mant, 1997, May. Epidemiology of genital prolapse: observations from the Oxford Family Panning Association Study. British Journal of Obstetrics and Gynaecology, 104(5), 579-585.
3. Wu JM, Vaughan CP, Goode PS, Redden DT, Burgio KL, Richter HE, Markland AD. Prevalence and trends of symptomatic pelvic floor disorders in U.S. women. Obstet Gynecol. 2014 Jan;123(1):141-148. doi: 10.1097/AOG.0000000000000057. PMID: 24463674; PMCID: PMC3970401.
What are the risks?
As with any device inserted into the vagina, there is the risk of infection and damage to vaginal/perineal tissues and the surrounding muscles. Materna Prep is an investigational medical device, so there may be risks that are unknown at this time. Materna Prep has been categorized as a non-significant risk device.
What are the benefits?
The purpose of the study is to determine if Materna Prep may be associated with these improved outcomes for mothers:
- Shorten delivery times
- Prevent pelvic floor muscle injury
- Minimize tearing
- Reduce the need for instruments used during deliveries (Use of vacuum or forceps to deliver your baby)
- Reduce need for C-Section
- Improve neonatal outcomes (the baby’s APGAR scores)

What does participation involve?
Informed Consent
If you are eligible, your doctor will discuss the potential benefits and risks of study participation and then ask if you are willing to participate in this study. If you decide to participate, you will be asked to sign an Informed Consent Form.
There’s no cost to participate. As a study participant, you may receive:
- Monetary compensation for your time completing the follow-up visit(s)
- Reimbursement for your travel expenses
- 3-month diaper subscription ($249 value) after each ultrasound visit
If you have any questions, please click the button below.
CAUTION: Investigational device. Limited by United States law to investigational use. Exclusively for clinical investigations. CL0063 Rev A.

